|
This model simulates the slaking of lime with green liquor. The chemical reactions are:
Slaking CaO + H2O ----> Ca (OH) 2
Causticising Ca (OH) 2 + Na2CO3 ----> CaCO3 + 2NaOH
The model calculates the extent of these reactions and the resulting changes in outlet stream composition and temperature. The makeup lime requirement is calculated by the block based on stoichiometry. A specified fraction of the solids are removed as grits. Vapor produced by heat of slaking is calculated.
|
What do you want to see?
|
 |
|
Data |
Unit |
Description |
|
Set |
Item |
Type |
Native |
|
Equipment Properties |
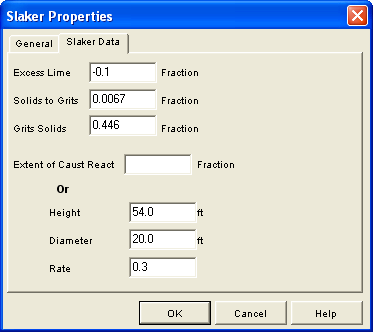
Height |
Length |
ft |
Height of cylindrical slaker. |
| Diameter |
Length |
ft |
Diameter of slaker. |
| ExcessLime |
Fraction or Percent |
Fraction |
Excess lime over the stoichiometric requirement for the causticising reaction, (typically -5 to 0%). |
| Rate |
|
|
A parameter to control the rate of forward reaction in causticising (default=1.0). |
| SolidsToGrits |
Fraction or Percent |
Fraction |
Fraction of the total solids in the slaker which are removed as grits. |
| GritsSolids |
Fraction or Percent |
Fraction |
The solids fraction of the grits. |
| ExtOfCaustReact |
Fraction or Percent |
Fraction |
|
Based on the feed rate of NaCO3 in the green liquor, the total lime requirement is calculated from stoichiometry. If necessary, the makeup lime flow is recalculated.
The slaking reaction is assumed to be proceeding to completion in the slaker unless the amount of water is insufficient for complete slaking. The heat of slaking is 285.4 kcal/kg and the corresponding rise in temperature of the liquor stream is calculated. Vaporization may occur if the temperature is high enough. Any vapor produced becomes the third outlet stream.
Significant causticising can occur in a slaker and the extent of reaction is computed from a model similar to the one presented in [1]. The slaker is assumed to be an ideal CSTR in which case the following equation applies.
V/F0 = X/Rc
where, the rate of causticising is given by,
Rc = k1( [NaCO3]0 (1-X) )a - k2(EA0+X [NaCO3]0 )b
k1 = Rf exp (-29.0/RT + 4.25)
k2 = exp (-25.6/RT - 8.3)
a = 1.0
b = 3.0
Nomenclature
V Volume of the CSTR
F0 Molar feed rate of sodium carbonate
X Fractional conversion of sodium carbonate
Rc Rate of causticising
[NaCO3]0 Initial sodium carbonate concentration g/l as Na2O
EA0 Initial Efffective alkali g/l as Na2O
R Gas constant
T Absolute temperature
Rf Rate of forward reaction (user specified, default = 1.0)
The exit conversion X is calculated iteratively from the above equations.
A fraction of the solids in the slaker is removed as grits. The grits stream solids are assumed to have the same composition as the suspended solids in the exit liquor stream.
 Missing Stream(s)!
Missing Stream(s)!
Solution: check if all the streams are connected to the equipment.